Your Partner for Japan Regulatory,
Development Strategy and Market Access
Development Strategy and Market Access
CoreMed is a Japan based Contract Regulatory and Strategy Development Consulting Firm specifically providing Non-Japanese pharmaceutical, bio-pharmaceutical and medical device companies with state-of-the-art strategic regulatory consulting services.
Comprehensive Support for Pharmaceutical Development
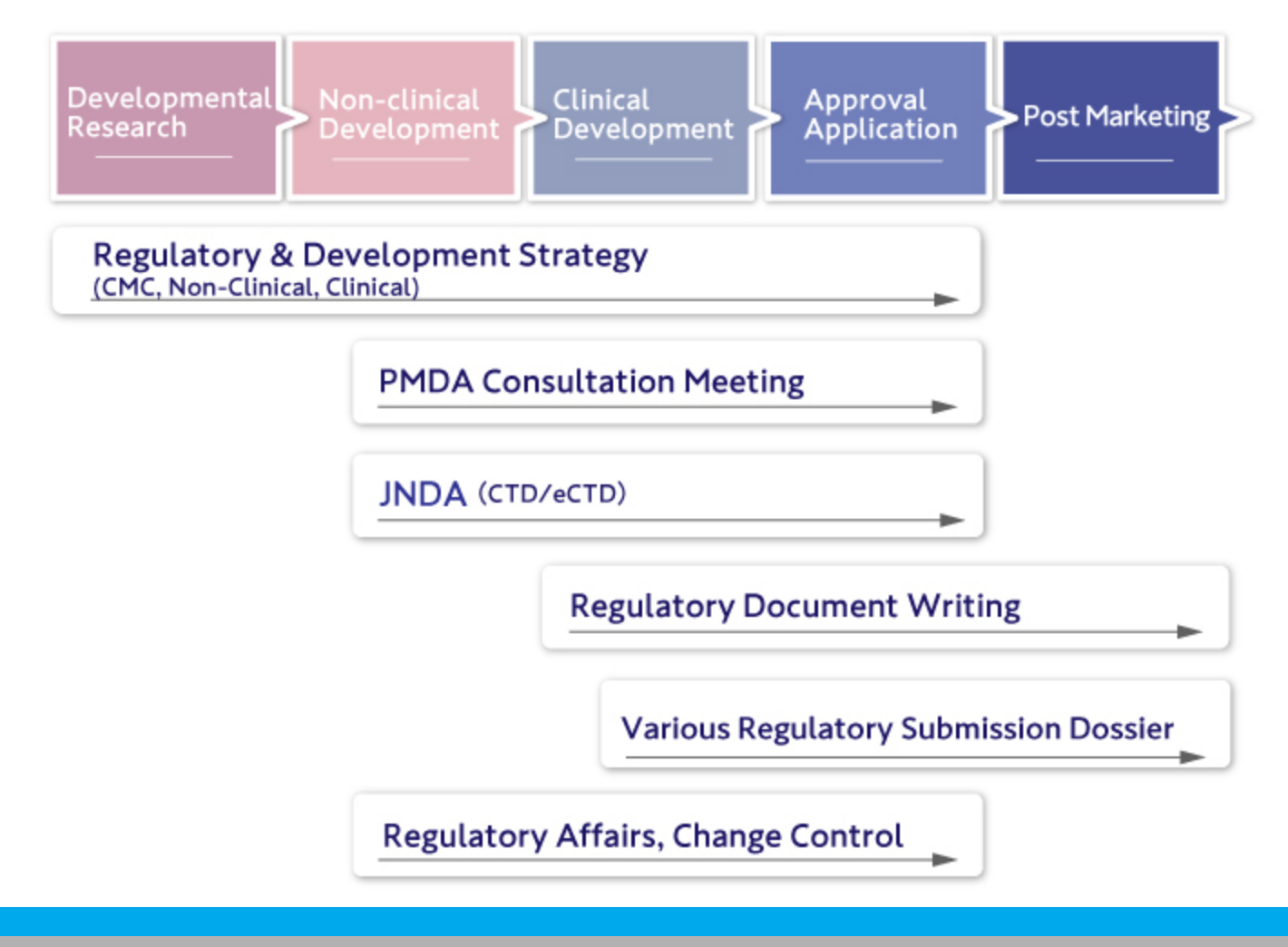
CoreMed offers comprehensive support for all development and regulatory tasks, strategy, PMDA consultation, regulatory submission strategies including CMC, non-clinical, and clinical, and the preparation of regulatory submission documents (CTD M1-M5/eCTD).
We handle various regulatory tasks associated with submissions and respond to inquiries through to approval.
We also manage changes post-application and approval.
All staff members at CoreMed are extraordinarily skilled, talented, creative, and experienced and grapple with projects toward a goal which is regulatory approval under Supreme Quality.
Our people at CoreMed are passionately supporting pharmaceutical and biopharmaceutical companies bring products to launch by designing development strategies, nonclinical and clinical strategies for pharmaceuticals and biopharmaceuticals including regenerative medicines.
> And More

Success Story
Witness the Success Stories of Our Clients through Collaboration with CoreMed
> And More